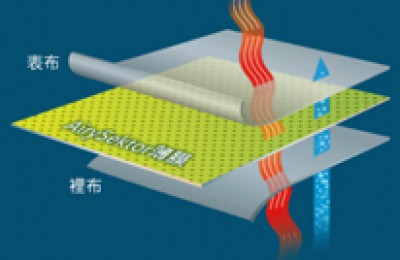
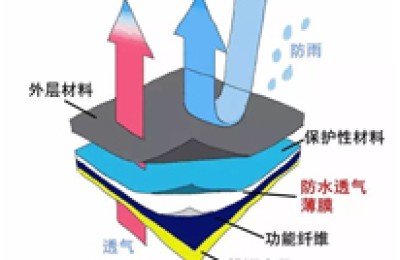
The chemical structure of polytetrafluoroethylene is formed by replacing all hydrogen atoms in polyethylene with fluorine atoms.
Its molecular formula is: The F atom in the PTFE molecule covers the C-C bond, and the C-F bond energy is high and particularly stable. It is not corroded by any chemicals except alkali metals and elemental fluorine.

The F atom in the PTFE molecule is symmetrical, and the two elements in C-F are covalently combined. There are no free electrons in the molecule, and the entire molecule is neutral.
This gives PTFE excellent dielectric properties. Because there are no gram bonds in the molecular structure of PTFE, its crystallinity is very high.
Because there is an inert fluorine-containing shell outside the PTFE molecule, it has outstanding non-stick properties and low friction coefficient.
Insulation: Not affected by the environment and frequency, the volume resistance can reach 1018 ohms?cm, the dielectric loss is small, and the breakdown voltage is high.
High and low temperature resistance: The effect on temperature does not change much, the temperature range is wide, and the usable temperature is -190~260℃.
Self-lubricating: It has the smallest friction coefficient among plastics and is an ideal oil-free lubricating material.
Surface non-stickiness: No known solid material can adhere to the surface. It is a solid material with the smallest surface energy.
Atmospheric aging resistance, radiation resistance and low permeability: the surface and performance remain unchanged after long-term exposure to the atmosphere.
Non-flammability: Oxygen limiting index is below 90. </p